- Research Article
- Open access
- Published:
Repetitive Stimulation of the Pituitary with Growth-Hormone-Releasing Hormone Alters the Proportion of 22 and 20 Kilodalton Human-Growth Hormone Released
International Journal of Pediatric Endocrinology volume 2010, Article number: 781317 (2010)
Abstract
Background/Aims. 20 Kilodalton-hGH (20 K-hGH) is the second most abundant pituitary GH variant after 22 K-hGH. In the steady state the proportion of 20 : 22 K-hGH appears constant; does this proportion change with repetitive somatotroph stimulation? Methods. Forty adult males were randomised to receive a GHRH(1–29) bolus
bolus 
 (
( or
or 
 (
( , preceded or followed by a saline bolus, 1 week apart. Four to six weeks later, 10 subjects received
, preceded or followed by a saline bolus, 1 week apart. Four to six weeks later, 10 subjects received 
 GHRH(1–29)
GHRH(1–29) at 0, 60, 120, and 180 minutes. Clearance rate of 22 and 20 K-hGH was measured in 10 subjects. Results. Total amount/proportion of 22 K-hGH/20 K-hGH secreted was similar for both GHRH(1–29)
at 0, 60, 120, and 180 minutes. Clearance rate of 22 and 20 K-hGH was measured in 10 subjects. Results. Total amount/proportion of 22 K-hGH/20 K-hGH secreted was similar for both GHRH(1–29) doses. Repetitive stimulation reduced the amount of 22 K-hGH released whereas the amount of 20 K-hGH did not change significantly leading to an increase in the proportion of 20 K-hGH
doses. Repetitive stimulation reduced the amount of 22 K-hGH released whereas the amount of 20 K-hGH did not change significantly leading to an increase in the proportion of 20 K-hGH  . Half-life of 20 and 22 K-hGH were not significantly different
. Half-life of 20 and 22 K-hGH were not significantly different  . Conclusions. Repetitive stimulation of the somatotroph may alter the proportion of GH variant released.
. Conclusions. Repetitive stimulation of the somatotroph may alter the proportion of GH variant released.
1. Introduction
Growth-hormone-releasing hormone (GHRH) acts on the GHRH receptor in the pituitary gland to alter the rate of GH gene transcription, increasing the amount of growth hormone produced and released [1]. GH secretion occurs immediately following a GHRH pulse, with the amount of GH released in response to an intravenous bolus dependent both on dose of GHRH and on how frequently the pituitary is stimulated with GHRH. Two main forms of GH account for most of the GH released in response to GHRH stimulation [2, 3], 22 K-hGH (191 amino acids) (approximately 75% of total secretion) and 20 K-hGH (176 amino acids) (5–10%) [4]. Numerous other forms are also detectable [5].
The 20 K-hGH version lacks residues 32–46 as a result of alternate splicing within exon 3, but retains high biological activity on the GH receptor. It may bind less tightly to the extracellular domain of the GH receptor, but appears to have the same efficacy at the full length receptor [6]. The physiological importance of the different isoforms of GH in humans remains unclear as sensitive and specific immunoassays for 20 K-hGH have only been recently developed [7]. 22 and 20 K-hGH have almost identical somatogenic activity in prepubertal male and female dwarf rats [8], but this reflects interaction with rodent receptors which differ from human GH receptor.
It is not known what controls the ratio of splicing of 20 versus 22 H-hGH products in the pituitary, and whether they are stored as efficiently, but when secretion is studied, the 22 K : 20 K-hGH ratio remains constant when measured over a 24 hour period and following exercise [9]. In children the percentage of the 20 K-hGH isoform remains constant independent of age, sex, puberty, height, body mass index, and rate of GH production [10]. These observations have led to the suggestion that the overall production of 20 K-hGH is under the same regulation as that of 22 K-hGH. However, recent in vitro studies using transgenic mice pituitary cell lines have specifically addressed the relative splicing efficiencies of GH products in the context of mutations that alter the usage of splice donor/acceptor sites in favour of a dominant negative 17.5 K-hGH form in which exon 3 sequences are completely eliminated [11]. In this case, driving synthesis and production in an in vitro system altered the relative amounts of alternately splice products made. We have, therefore, evaluated the impact of repetitive stimulation with GHRH in humans, a stimulus that also directly drives GH synthesis, on the amount of 20 : 22 K-hGH produced.
2. Subjects and Methods
2.1. Subjects
40 adult male volunteers aged 19–25 years were recruited to the study. All were of normal height (170–182 cm), weight (68–80 kg), and body mass index (22.8–24.4). None of the subjects smoked and alcohol was avoided on the day before each study. Subjects fasted from midnight prior to admission the following morning to the Clinical Investigations Unit at 08.00h. An intravenous (iv) cannula was placed in a forearm vein to allow blood sampling and administration of iv bolus injections of GHRH(1–29)NH2 (Pfizer, Stockholm, Sweden). Subjects were allowed to rest for 60 minutes before the study commenced. Exercise and daytime napping were prohibited.
Study 1 (Dose and Intensity Studies)
The 40 subjects were randomized to receive either a GHRH(1–29)NH2 bolus (0.5  g/kg (
g/kg ( ) or 1.0
) or 1.0  g/kg (
g/kg ( )), preceded or followed by an IV bolus injection of saline. The two studies were separated in time by an interval of 1 week (Study 1).
)), preceded or followed by an IV bolus injection of saline. The two studies were separated in time by an interval of 1 week (Study 1).
Ten of the subjects who originally received a 0.5  g/kg GHRH(1–29)NH2 bolus were chosen at random to participate in a second study. On a separate occasion, 4–6 weeks after the dose studies, these individuals received 0.5
g/kg GHRH(1–29)NH2 bolus were chosen at random to participate in a second study. On a separate occasion, 4–6 weeks after the dose studies, these individuals received 0.5  g/kg GHRH(1–29)NH2 administered at 0, 60, 120, and 180 minutes, to determine within group and within individual variation in response to GHRH(1–29)NH2 stimulation, with the 20 and 22 kDa responses 60 minutes after the first GHRH injection used to determine within group and within individual variation in response to GHRH(1–29)NH2 stimulation (Reproducibility Study). Blood samples were drawn at 10 minutes intervals for the measurement of serum 20 and 22 K-hGH until 240 minutes. At the start of the study an additional sample was drawn for the measurement of insulin-like growth factor-1 (IGF-1). Samples were spun and separated and the serum stored at −20
g/kg GHRH(1–29)NH2 administered at 0, 60, 120, and 180 minutes, to determine within group and within individual variation in response to GHRH(1–29)NH2 stimulation, with the 20 and 22 kDa responses 60 minutes after the first GHRH injection used to determine within group and within individual variation in response to GHRH(1–29)NH2 stimulation (Reproducibility Study). Blood samples were drawn at 10 minutes intervals for the measurement of serum 20 and 22 K-hGH until 240 minutes. At the start of the study an additional sample was drawn for the measurement of insulin-like growth factor-1 (IGF-1). Samples were spun and separated and the serum stored at −20 C prior to assay.
C prior to assay.
Study 2 (Half-Life Study)
Following Study 1, 10 further (i.e., different) subjects chosen at random received a single iv bolus injection of 0.5  g/kg GHRH(1–29)NH2 followed by serial blood sampling to estimate the half life of endogenously released 20 and 22 K-hGH. This study was undertaken 6–8 weeks after the completion of Study 1. In this study an additional iv cannula was inserted in the contralateral arm for infusion of Somatostatin (1–14) (Ferring Pharmaceuticals, Malmo, Sweden) (20
g/kg GHRH(1–29)NH2 followed by serial blood sampling to estimate the half life of endogenously released 20 and 22 K-hGH. This study was undertaken 6–8 weeks after the completion of Study 1. In this study an additional iv cannula was inserted in the contralateral arm for infusion of Somatostatin (1–14) (Ferring Pharmaceuticals, Malmo, Sweden) (20  g/m2/hour). Blood samples were drawn at 10 minutes intervals for the first 60 minutes following the bolus administration of GHRH(1–29)NH2. At this point the somatostatin (1–14) infusion was commenced and blood samples drawn at 5 minutes intervals for the following 90 minutes and assayed as in Study 1.
g/m2/hour). Blood samples were drawn at 10 minutes intervals for the first 60 minutes following the bolus administration of GHRH(1–29)NH2. At this point the somatostatin (1–14) infusion was commenced and blood samples drawn at 5 minutes intervals for the following 90 minutes and assayed as in Study 1.
These studies were approved by the ethics committee's at University College London Hospitals and University College London, and written informed consent obtained from the subjects.
3. Assays
3.1.  K-hGH Assay
K-hGH Assay
20 K-hGH was measured by a triple antibody ELISA. Microtitre plates were coated for 7 hours at 37 C with a 20 K-hGH specific mouse monoclonal antibody HGH33 in phosphate buffered saline (PBS) [12]. Prior to assay, nonspecific binding sites were blocked with 5% milk protein for 45 minutes. Standards and samples were incubated in triplicate for 90 minutes, after which a polyclonal sheep anti-hGH antibody (Scottish Antibody Production Unit, Roslin, UK) was bound to the 20 K-hGH33 complex. This was followed by the addition of a biotinylated rabbit antigoat antiserum (DAKO Ltd., High Wycombe, UK) which recognises sheep immunoglobulins. Visualisation used an avidin:biotin complex with alkaline phosphatase as enzyme tracer (DAKO Ltd, High Wycombe, UK). The plate was developed at 24
C with a 20 K-hGH specific mouse monoclonal antibody HGH33 in phosphate buffered saline (PBS) [12]. Prior to assay, nonspecific binding sites were blocked with 5% milk protein for 45 minutes. Standards and samples were incubated in triplicate for 90 minutes, after which a polyclonal sheep anti-hGH antibody (Scottish Antibody Production Unit, Roslin, UK) was bound to the 20 K-hGH33 complex. This was followed by the addition of a biotinylated rabbit antigoat antiserum (DAKO Ltd., High Wycombe, UK) which recognises sheep immunoglobulins. Visualisation used an avidin:biotin complex with alkaline phosphatase as enzyme tracer (DAKO Ltd, High Wycombe, UK). The plate was developed at 24 C over 15–19 hours using p-nitrophenylphosphate as substrate (Sigma-Aldrich Co. LTD, Poole, UK) with the colour reaction measured at 405 nm.
C over 15–19 hours using p-nitrophenylphosphate as substrate (Sigma-Aldrich Co. LTD, Poole, UK) with the colour reaction measured at 405 nm.
Recombinant methionyl-20 K-hGH (Genentech, San Francisco, CA, USA) (dissolved in adult human standard matrix) was used as standard at concentrations up to 10 ng/mL. The minimum detection limit of the assay was 0.1 ng/mL as determined by the mean of zero + 3SD, based on 25 repeat determinations. The within-assay coefficients of variance (CV) were 12% and 6.5% at 2.8 and 7.0 ng/mL, respectively. The between-assay CV were 13.0% and 11.8% at 2.8 and 5.3 ng/mL, respectively. The ELISA showed no detectable cross reactivity with recombinant 22 K-hGH (International Reference Preparative 88/624; NIBSC, South Mimms, UK) at physiological concentrations (50–100 ng/mL). At very high concentrations of 22 K-hGH (10  g/mL) cross reactivity was 0.6%. At physiological concentrations, pituitary-derived hGH standard (International Standard 80/505; NIBSC) (50–100 ng/mL) showed 3%-4% cross-reactivity in the 20 K-hGH assay. There was no cross reactivity at pathological concentrations of hPRL (4000 mU/L; North East Thames Region Immunoassay Service, St Bartholomew's Hospital, London, UK).
g/mL) cross reactivity was 0.6%. At physiological concentrations, pituitary-derived hGH standard (International Standard 80/505; NIBSC) (50–100 ng/mL) showed 3%-4% cross-reactivity in the 20 K-hGH assay. There was no cross reactivity at pathological concentrations of hPRL (4000 mU/L; North East Thames Region Immunoassay Service, St Bartholomew's Hospital, London, UK).
3.2. 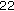 K-hGH Assay
K-hGH Assay
Serum 22 K-hGH was measured using the Hybritech Tandem-R immunoradiometric assay (Hybritech, Liege Belgium). This is highly specific for the 22 K-hGH variant of hGH and showed no cross-reactivity with 20 K-hGH. The minimum detection limit of the assay was 0.19 ng/mL. The within-assay CV were 10.6%, 5.2% and 4.9% at 0.5, 5.5, and 10.2 ng/mL. The between-assay CV were 15.4%, 8.0%, and 6.3% at 1.6, 5.2, and 13.8 ng/mL. The assay was standardized against HS2243E (NIH, Bethesda, Maryland, USA) and has been recalibrated against International Standard 80/505 (NIBSC, South Mimms, UK).
3.3. IGF-I Assay
IGF-I was measured by a commercial immunoradiometric assay (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). This is a nonextraction method where IGF binding proteins are separated from the IGF-I by acidification of the sample and excess of IGF-II is added to block the binding proteins from recombining with the IGF-I. The within-assay CV were 4.6% and 3.3% at 6.1 and 292.5 ng/mL, respectively. The between-assay CV were 15.5% and 11.3% at 88.6 and 240.4 ng/mL. The standards were prepared from recombinant IGF-I and calibrated against International Reference Preparation 87/518 (NIBSC, South Mimms, UK). The minimum detection limit of the assay was 6 ng/mL.
4. Statistics
All data were explored for the normality of their distribution and when appropriate natural-log (Ln) transformed. For this study, "total" GH refers to the sum of the 22 and 20 K-hGH serum concentrations. The effect of the dose of GHRH(1–29)NH2 on the amount and proportion of the GH isoforms was compared using paired Student's  -test as were the group data obtained from the stimulation tests conducted on the two separate occasions 4–6 weeks apart. One way Analysis of Variance (ANOVA) with a repeated measures design was used for the study in which GHRH(1-29)NH2 was administered thrice. The coefficient of variation was estimated for the within individual variation for percentage of 20 K-hGH secreted in response to GHRH(1–29)NH2 stimulation. Multiple linear regression was used to explore the relationship between the percentage of 20 K-hGH released and factors such as intensity of response. The half-lives of 22 and 20 K-hGH were determined using techniques previously described by ourselves for GH [13].
-test as were the group data obtained from the stimulation tests conducted on the two separate occasions 4–6 weeks apart. One way Analysis of Variance (ANOVA) with a repeated measures design was used for the study in which GHRH(1-29)NH2 was administered thrice. The coefficient of variation was estimated for the within individual variation for percentage of 20 K-hGH secreted in response to GHRH(1–29)NH2 stimulation. Multiple linear regression was used to explore the relationship between the percentage of 20 K-hGH released and factors such as intensity of response. The half-lives of 22 and 20 K-hGH were determined using techniques previously described by ourselves for GH [13].
5. Results
5.1. General
Data were available for all individuals who participated in the dose study. Nine of the 10 subjects who were selected for the triple GHRH(1–29)NH2 stimulation study completed all three stimulation tests ((a) Saline, (b) 0.5  g/kg baseline, (c) 0.5
g/kg baseline, (c) 0.5  g/kg 4–6 weeks later at 0, 60, 120, and 180 minutes), (one individual only completed the 0 and 60 minutes time points in the triple GHRH(1–29)NH2 study).
g/kg 4–6 weeks later at 0, 60, 120, and 180 minutes), (one individual only completed the 0 and 60 minutes time points in the triple GHRH(1–29)NH2 study).
Following the bolus injection of saline there was no change in the serum 22 or 20 K-hGH concentrations which remained low and close to the detection limit of both assays. In all studies both 22 and 20 K-hGH concentrations were detectable above the lower limit of detection of the assay. Serum IGF-1 concentrations were 300 ng/mL (range 220–375) in the individuals at the start of the study.
5.2. Study 1
There was no significant difference in the peak response to different doses of GHRH(1–29)NH2 in the total amounts of 22 K-hGH (mean peak 22 K-hGH to 0.5  g/kg GHRH(1–29)NH2 20.8 ± 3.4 ng/mL and to 1.0
g/kg GHRH(1–29)NH2 20.8 ± 3.4 ng/mL and to 1.0  g/kg GHRH(1–29)NH2 22.3 ± 2.3 ng/mL; P = .37: 20 K-hGH (2.6 ± 1.5 ng/mL and 2.2 ± 0.4 ng/mL, respectively;
g/kg GHRH(1–29)NH2 22.3 ± 2.3 ng/mL; P = .37: 20 K-hGH (2.6 ± 1.5 ng/mL and 2.2 ± 0.4 ng/mL, respectively;  ) or the proportion of 20 K-hGH (10.7 ± 2.5% and 9.4 ± 1.6%, respectively;
) or the proportion of 20 K-hGH (10.7 ± 2.5% and 9.4 ± 1.6%, respectively;  ) secreted. However, the higher the peak hGH concentration, irrespective of dose, the greater the percentage of 20 K-hGH was present (
) secreted. However, the higher the peak hGH concentration, irrespective of dose, the greater the percentage of 20 K-hGH was present ( ;
;  ).
).
We tested the effects of the GHRH(1–29)NH2 dose administered as a single and then further bolus injections and determined, the amounts and proportion of 20 K-hGH released. With more frequent stimulation the amount of 22 K-hGH released decreased significantly (One way ANOVA  ;
;  ) whereas the amount of 20 K-hGH did not change significantly, resulting in a net increase in percentage of 20 K-hGH present (
) whereas the amount of 20 K-hGH did not change significantly, resulting in a net increase in percentage of 20 K-hGH present ( ;
;  ) (Figure 1, Table 2 and Figure 2). By multiple regression analysis (
) (Figure 1, Table 2 and Figure 2). By multiple regression analysis ( ;
;  ) the intensity of response to GHRH(1–29)NH2 was the major determinant of the percentage of 20 K-hGH present (B = 3.89,
) the intensity of response to GHRH(1–29)NH2 was the major determinant of the percentage of 20 K-hGH present (B = 3.89,  ) with a much lesser contribution from the total amount of hGH released (B = 0.11,
) with a much lesser contribution from the total amount of hGH released (B = 0.11,  ).
).
The within individual coefficients of variation for 22 K-hGH, 20 K-hGH, and the percentage of 20 K-hGH secreted following GHRH(1–29)NH2 administration on the two occasions were 19.0 ± 4.4, 24.5 ± 9.2, and 20.5 ± 6.7%, respectively. Group data were similar on the two occasions with no significant differences detected (Table 1).
 g GHRH(1–29)NH2 stimulation in 10 subjects on two separate occasions (4–6 weeks apart) on 22, 20 kilodalton and percentage of 20 kilodalton human-growth hormone secreted (concentrations measured 60 minutes after administration of GHRH(1–29)NH2).
g GHRH(1–29)NH2 stimulation in 10 subjects on two separate occasions (4–6 weeks apart) on 22, 20 kilodalton and percentage of 20 kilodalton human-growth hormone secreted (concentrations measured 60 minutes after administration of GHRH(1–29)NH2). g GHRH(1–29)NH2.
g GHRH(1–29)NH2.5.3. Study 2
The initial half-lives of clearance of 22 K-hGH (14.2 ± 1.1 minutes) and 20 K-hGH (15.7 ± 0.9 minutes) were not significantly different ( ) in these subjects.
) in these subjects.
6. Discussion
These data demonstrate that the intensity of response to GHRH(1–29)NH2 appears to be a more important determinant of the amount of 20 K-hGH released than the dose of GHRH(1–29)NH2 administered. In response to a repeated GHRH(1–29)NH2 stimulation lower total concentrations of hGH were achieved and a higher percentage of 20 K-hGH was present (Figure 1, Table 2 and Figure 2). These findings have implications for clinical practice as many endocrine centres measure peak 22 K-hGH response to two consecutive GH provocation tests to diagnose growth hormone deficiency.
The overall effect of GHRH(1–29)NH2 on total hGH secretion was to reduce the concentrations achieved when the stimulus was applied with an interval of 1, 2, or 3 hours compared to the initial application of GHRH(1–29)NH2 a finding consistent with the literature [14]. This effect was more marked for 22 K-hGH than 20 K-hGH. Our study differs from previous reports in that we have used a fixed dose of GHRH(1–29)NH2 and measured the concentration of GH variants not only in the steady state but also in response to intensive repeat stimulation [10]. We confirm that in the steady state with minimum stimulation the proportion of 20 K-hGH found in the circulation accounts for approximately 10% of total, and that after repeated stimulation 20 K-hGH comprises 15.7% of total hGH released. Our findings clarify previous reports which have also addressed the idea that repeated stimulation may affect the proportions of the different GH isoforms produced and released, but have been unable to distinguish isoforms produced by splicing, from other minor forms that can arise from fragmentation or deamidation, included in "non-22 K-hGH" isoforms present [15].
The effect of repeated stimulation with GHRH(1–29)NH2 on the percentage of 20 K-hGH present in the circulation is unlikely to be materially affected by differences in the clearance of 20 K-hGH as the measured disappearance half-lives of 22 K-hGH and 20 K-hGH were similar in these subjects. This does not rule out the possibility that there could be differential binding to circulating growth hormone binding proteins in the steady state, but this is less likely to impact on our findings given the short-time frames over which repeated stimulation was performed. It is more likely that our results reflect differences in the ratio of 20 versus 22 K-hGH products secreted under these conditions. In addition to the factors altering splicing efficiency, differences in protein stability, granule packaging, or secretion could all contribute to these differences, as could the relative release rates of different granule pools, containing newly synthesized versus previously synthesized GH [16]. It is however important to note that differences in the sensitivities of the 20 and 22 K-hGH assays may also impact on these results with the larger decline in 22 K-hGH which was detected possibly reflecting the difference between these two assays especially at values close to the limit of detection of the assay.
Although the biological effects of these changes may be insignificant, these observations nevertheless have implications for interpreting provocative GH testing in clinical practice. Many guidelines for testing the GH axis suggest that more than one test is undertaken [1]. In many clinical situations it is convenient to combine tests so that the two tests may be applied consecutively. Leaving aside whether this is statistically valid or not [17] there is the potential problem that the "cut-off" used to define normality on the second test should not necessarily be the same as that used for the first, especially if the components of what is being measured is changing between tests. With an increasing move towards monoclonal GH immunoassays and calibration using 22 K biosynthetic hGH it is obvious that total bioactive GH secretion will be greater than that estimated only for 22 K-hGH. This may become important in situations of poor GH storage (with inadequate somatotroph mass) or constant hyperstimulation, where both reduction in secretory reserve and an increase in the proportion of 20 K-hGH and other isoforms, may rise as hGH rises. Our observations stress the importance of considering the impact of chronic or repetitive stimulation of the GH axis on the production of GH bioactive forms that may escape detection in clinical diagnostic tests that specifically measure only maximal 22 K-hGH secretion. These observations may have important implications for the interpretation of clinical diagnostic tests of the GH axis.
References
Attie KM, Bengtsson B-A, Blethen SL: Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH research society. Journal of Clinical Endocrinology and Metabolism. 2000, 85 (11): 3990-3993. 10.1210/jc.85.11.3990.
Baumann G: Growth hormone heterogeneity in human pituitary and plasma. Hormone Research. 1999, 51 (supplement 1): 2-6. 10.1159/000053128.
DeNoto FM, Moore DD, Goodman HM: Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Research. 1981, 9 (15): 3719-3730. 10.1093/nar/9.15.3719.
Chapman GE, Rogers KM, Brittain T: The 20,000 molecular weight variant of human growth hormone. Preparation and some physical and chemical properties. Journal of Biological Chemistry. 1981, 256 (5): 2395-2401.
Baumann G, Stolar MW, Amburn K: Molecular forms of circulating growth hormone during spontaneous secretory episodes and in the basal state. Journal of Clinical Endocrinology and Metabolism. 1985, 60 (6): 1216-1220. 10.1210/jcem-60-6-1216.
Ikeda M, Matsumoto K, Uchida H: Cellular activities of 20K- and 22K-hGH do not necessarily correlate with their binding affinities for rat GH receptor. Hormone Research. 2000, 54 (3): 136-142. 10.1159/000053247.
Tsushima T, Katoh Y, Miyachi Y: Serum concentration of 20K human growth hormone (20K hGH) measured by a specific enzyme-linked immunosorbent assay. Journal of Clinical Endocrinology and Metabolism. 1999, 84 (1): 317-322. 10.1210/jc.84.1.317.
Ishikawa M, Tachibana T, Kamioka T, Horikawa R, Katsumata N: Comparison of the somatogenic action of 20 kDa- and 22 kDa-human growth hormones in spontaneous dwarf rats. Growth Hormone and IGF Research. 2000, 10 (4): 199-206. 10.1054/ghir.2000.0153.
Wallace JD, Cuneo RC, Bidlingmaier M: The response of molecular isoforms of growth hormone to acute exercise in trained adult males. Journal of Clinical Endocrinology and Metabolism. 2001, 86 (1): 200-206. 10.1210/jc.86.1.200.
Ishikawa M, Yokoya S, Tachibana K: Serum levels of 20-kilodalton human growth hormone (GH) are parallel those of 22-kilodalton human GH in normal and short children. Journal of Clinical Endocrinology and Metabolism. 1999, 84 (1): 98-104. 10.1210/jc.84.1.98.
Salemi S, Yousefi S, Lochmatter D: Isolated autosomal dominant growth hormone deficiency: stimulating mutant GH-1 gene expression drives GH-1 splice-site selection, cell proliferation, and apoptosis. Endocrinology. 2007, 148 (1): 45-53. 10.1210/en.2006-0772.
Mellado M, Miguel Rodriguez-Frade J, Kremer L, Martinez-Alonso C: Characterization of monoclonal antibodies specific for the human growth hormone 22K and 20K isoforms. Journal of Clinical Endocrinology and Metabolism. 1996, 81 (4): 1613-1618. 10.1210/jc.81.4.1613.
Hindmarsh PC, Matthews DR, Brain CE: The half-life of exogenous growth hormone after suppression of endogenous growth hormone secretion with somatostatin. Clinical Endocrinology. 1989, 30 (4): 443-450. 10.1111/j.1365-2265.1989.tb00444.x.
Suri D, Hindmarsh PC, Matthews DR, Brain CE, Brook CGD: The pituitary gland is capable of responding to two successive doses of growth hormone releasing hormone (GHRH). Clinical Endocrinology. 1991, 34 (1): 13-17. 10.1111/j.1365-2265.1991.tb01729.x.
Coya R, Algorta J, Boguszewski CL: Circulating non-22 kDa growth hormone isoforms after a repeated GHRH stimulus in normal subjects. Growth Hormone and IGF Research. 2005, 15 (2): 123-129. 10.1016/j.ghir.2004.09.002.
Duncan RR, Greaves J, Wiegand UK: Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003, 422 (6928): 176-180. 10.1038/nature01389.
Guyatt G: Evaluating diagnostic tests. Clinical Epidemiology: How To Do Clinical Practice Research. Edited by: Sackett D, Haynes B. 2005, Lippincott, Williams and Wilkins, Philidelphia, Pa, USA, 273-322.
Acknowledgment
The authors thank Mrs. Brankica Leonard and Mrs. Caroline Dance for their technical support and Professor Mario Mellado, Centro National de Biotecnologia, Universidad Autonoma Madrid, for the supply of the 20 K-hGH antibodies for the immunoassay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Webb, E.A., Pringle, P.J., Robinson, I.C.A.F. et al. Repetitive Stimulation of the Pituitary with Growth-Hormone-Releasing Hormone Alters the Proportion of 22 and 20 Kilodalton Human-Growth Hormone Released. Int J Pediatr Endocrinol 2010, 781317 (2010). https://doi.org/10.1155/2010/781317
Received:
Accepted:
Published:
DOI: https://doi.org/10.1155/2010/781317
 K-hGH Assay
K-hGH Assay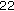 K-hGH Assay
K-hGH Assay
